

A Haworth projection doesn't try to do that. The chair drawing or "diamond lattice projection" shows these relationships pretty well. Also, substituents on the atoms in the ring can be found above the ring, below the ring, or sticking out around the edge of the ring. The chair drawing shows that relationship, but in a Haworth projection, the ring is drawn as though it were flat. For example, in a six-membered ring, the atoms in the ring adopt a zig-zag, up-and-down pattern in order to optimize bond angles. The most common way of drawing these rings are in "Haworth projections." Haworth projections don't reflect the real shape of the ring. Five-membered rings are called "furanoses" and six-membered rings are called "pyranoses". As a result, five- and six-membered rings are very common in sugars. The hemiacetal forms when a hydroxyl group along the carbon chain reaches back and bonds to the electrophilic carbonyl carbon. That's because the carbonyl is usually "masked" as a hemiacetal. In fact, if you have seen drawings of sugars before, you might not have noticed the carbonyl. Can a sugar react with itself? Of course. In just one molecule, we have both an electrophile (the carbonyl) and a number of nucleophiles (the hydroxyls). This sort of structure presents lots of possibilities for reactions. In general, a sugar is a molecule that contains an aldehyde or a ketone, and every carbon other than the carbonyl carbon has a hydroxyl group attached to it. One strict definition of a carbohydrate is a polyhydroxylated aldehyde or ketone. The term "sugar" is often applied to any simple carbohydrate. They carry specialized chemical reagents into enzymes where reactions essential to life are carried out. They aid in homebody security and defense operations by forming molecular codes on the surfaces of cells that identify whether the cell is one of our own or an intruder. For example, they lend structural support in the backbone of DNA.
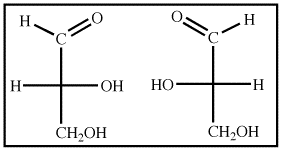
Although their best-known role is in energy storage in the form of glucose and starch, carbohydrates play a number of other roles. Β form: The configuration of a cyclic monosaccharide where the oxygen attached to the anomeric carbon is on the same side of the ring as the CH₂ OH group.\)Ĭarbohydrates are an important class of biological molecules. Α form: The configuration of a cyclic monosaccharide where the oxygen attached to the anomeric carbon is on the opposite side of the ring from the CH₂ OH group. Stereoisomers or optical isomers: Molecules that differ three-dimensionally by the placement of substituents around one or more atoms in a molecule.Ĭhiral carbon (asymmetric carbon):A carbon that is attached to four different types of atoms or groups of atoms.Īnomeric carbon: A carbon derived from the carbonyl carbon (the ketone or aldehyde functional group) of the open-chain form of the carbohydrate molecule. The α form of glucose has the anomeric OH group opposite from the CH₂OH group, while the β form has the anomeric OH group on the same side as the CH₂OH group.Įpimers: A type of stereoisomer that differs in configuration at a single stereogenic center (the anomeric carbon).Īnomers: A type of stereoisomer that differs in configuration at the hemiacetal or acetal carbon they are a specific type of epimer.Anomers differ in position at the anomeric carbon they are a special type of epimer.Epimers differ in the position of the atoms attached at one chiral carbon.The β form has the anomeric OH group on the same side as the CH₂ OH. The α form has the anomeric OH group at C-1 on the opposite side of the ring from the CH₂ OH group at C-5. For example, α-D-glucose and β-D-glucose below are anomers. The carbon atom that forms the new chiral center (C-1) is called the anomeric carbon.Īnomers are special cases of epimers that differ in position at the anomeric carbon in particular. When a molecule such as glucose converts to a cyclic form, it generates a new chiral center at C-1. Glucose and mannose are epimers that differ at the C-2 carbon, while glucose and galactose are epimers that differ at the C-4 carbon, as shown below. In the examples below, the difference in the position of the hydroxyl (OH) at one chiral carbon creates a pair of epimers. Epimers and anomers are types of stereoisomers of carbohydrates that differ in the position at a single carbon atom.Įpimers are stereoisomers that differ in the configuration of atoms attached to a chiral carbon.


 0 kommentar(er)
0 kommentar(er)
